The global demand for peptides has surged in recent years, driven by their potential in a wide array of research and therapeutic applications. As a result, many researchers and companies are sourcing these peptides from international markets, including China. However, buying peptides from overseas can sometimes be akin to navigating a minefield, with potential contaminants lurking beneath the surface. This blog post aims to shed light on some of the possible contaminants that have been found in peptides sourced from overseas.
Microbial Contamination
One of the most common types of contamination in peptides is microbial contamination. This can occur due to inadequate storage practices or during the synthesis process. Microbial contamination can pose serious safety concerns and lead to inaccurate experimental results or adverse effects when used in vivo.
Chemical Contamination
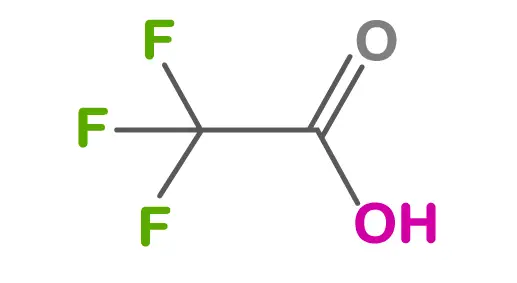
Chemical contaminants can also be a concern in peptides. These can include residual solvents or reagents used during the peptide synthesis process. In some cases, degradation of peptides during improper storage can lead to the formation of impurities or toxic byproducts.
Cross-Contamination
Cross-contamination with other peptides is another potential risk. This can occur when peptides are synthesized or packaged in facilities that handle multiple peptides. Even trace amounts of another peptide can interfere with experimental results or therapeutic applications.
Misidentified Peptides
In some cases, the peptide itself may be misidentified or misrepresented. This can occur due to errors in synthesis or labeling, leading to the distribution of a peptide that is different from what is claimed.
The Role of Quality Testing
Given these potential risks, quality testing is crucial when sourcing peptides from overseas. At Forever Young Pharmacy, we employ a range of analytical techniques to determine the purity and identity of peptides, including High-Performance Liquid Chromatography (HPLC) and mass spectrometry. These techniques provide valuable information about the composition and potential contaminants present in peptides, allowing for informed decisions regarding their use in various applications.
In conclusion, while buying peptides from overseas can offer cost and availability advantages, it’s crucial to be aware of the potential risks associated with contaminants. By understanding these risks and implementing rigorous quality testing measures, researchers and companies can ensure the safety and efficacy of the peptides they source from overseas.