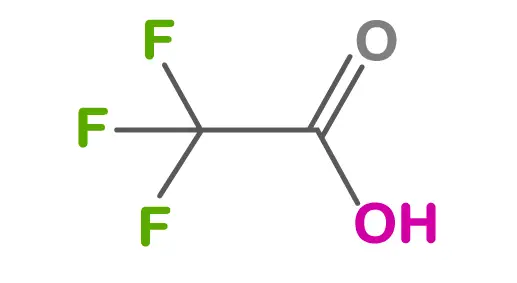
Peptides have become increasingly popular in various fields, including pharmaceuticals, cosmetics, and research. These short chains of amino acids play crucial roles in numerous biological processes and have shown promise in treating various medical conditions. However, ensuring the purity and safety of peptide samples as well as any drug product is paramount. One of the critical aspects of quality control is testing for bacterial or fungal contamination. In this blog post, we will explore the importance of such testing and the methods used to detect contaminants.
Why Testing for Contamination is Crucial
- Health and Safety Contaminated peptides can pose significant health risks. Bacterial and fungal contaminants can cause infections, allergic reactions, and other adverse effects. For instance, endotoxins produced by Gram-negative bacteria can lead to severe inflammatory responses and even septic shock. Ensuring that peptide samples are free from such contaminants is essential to protect the health and safety of end-users.
- Efficacy and Reliability The presence of contaminants can compromise the efficacy of peptides. Bacterial and fungal contaminants can interfere with the intended biological activity of the peptide, leading to unreliable results in research and reduced effectiveness in therapeutic applications. By testing for contamination, researchers and manufacturers can ensure that their peptide products perform as expected.
- Regulatory Compliance Regulatory agencies, such as the FDA and EMA, have stringent guidelines for the quality and safety of pharmaceutical products. Testing for bacterial and fungal contamination is a critical component of these guidelines. Compliance with these regulations is necessary to gain approval for peptide-based drugs and to maintain the trust of consumers and stakeholders.
- Product Shelf Life Contaminants can accelerate the degradation of peptides, reducing their shelf life. Bacterial and fungal growth can lead to the breakdown of peptide bonds and the formation of harmful by-products. Regular testing helps identify and eliminate contaminants, thereby extending the shelf life of peptide products.
Methods for Testing Bacterial and Fungal Contamination
- Bacterial Culture Bacterial culture is a traditional method for detecting bacterial contamination. The peptide sample is inoculated onto nutrient agar plates and incubated to allow bacterial growth. The presence of bacterial colonies indicates contamination. This method is highly sensitive and can identify a wide range of bacterial species.
- Fungal Culture Similar to bacterial culture, fungal culture involves inoculating the peptide sample onto Sabouraud’s dextrose agar plates and incubating them at a suitable temperature. The growth of fungal colonies indicates contamination. This method is effective for detecting yeasts and molds.
- Gram Stain Gram staining is a rapid method for identifying bacterial contamination. The peptide sample is stained with crystal violet and safranin, allowing the differentiation of Gram-positive and Gram-negative bacteria under a microscope. This method provides quick results and helps guide further testing.
- Endotoxin Testing The Limulus Amebocyte Lysate (LAL) assay is used to detect endotoxins, which are toxins produced by Gram-negative bacteria. The peptide sample is mixed with the LAL reagent, and the formation of a gel clot indicates the presence of endotoxins. This test is highly sensitive and specific.
- Molecular Techniques Polymerase Chain Reaction (PCR) and other molecular techniques can be used to detect specific bacterial and fungal DNA in peptide samples. These methods are highly sensitive and can identify contaminants that may not be detectable by culture methods.
- Microscopy Microscopic examination of peptide samples can reveal the presence of bacterial and fungal cells. Staining techniques, such as potassium hydroxide (KOH) preparation and fluorescent staining, enhance the visibility of contaminants under the microscope.
Conclusion
Testing peptides for bacterial and fungal contamination is a critical aspect of quality control that ensures the safety, efficacy, and reliability of peptide products. By employing a combination of traditional and modern testing methods, researchers and manufacturers can detect and eliminate contaminants, thereby protecting the health of end-users and complying with regulatory standards. As the use of peptides continues to grow, rigorous testing for contamination will remain essential to maintaining the highest standards of product quality.

Your blog has become an indispensable resource for me. I’m always excited to see what new insights you have to offer. Thank you for consistently delivering top-notch content!
Hey people!!!
HAVE A NICE DAY
Hello.
Good cheer to all on this beautiful day!!!!!
Good luck 🙂