Trifluoroacetic acid (TFA), a compound with a tongue-twisting name, plays a critical role in peptide chemistry. Whether you’re a seasoned researcher or just curious about the science behind peptides, let’s dive into the world of TFA and explore its significance.
What Is TFA?
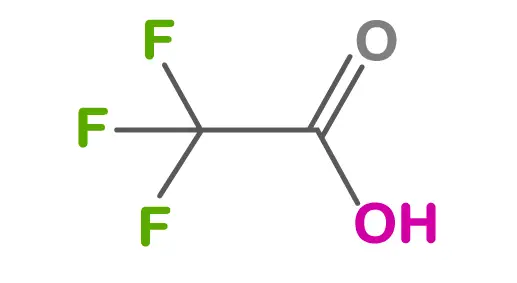
TFA (chemical formula: CF₃COOH) consists of three fluorine atoms attached to a central carbon atom. It’s a strong organic acid with unique properties.
Peptide Synthesis:
TFA is commonly used during solid-phase peptide synthesis (SPPS). In SPPS, peptides are assembled step by step on a solid support (resin). When the peptide chain is complete, TFA is applied to release the peptide from the resin. Think of it as the “unlock” button that frees the peptide from its molecular prison.
Why Is TFA There?
During SPPS, amino acids are protected with specific groups to prevent unwanted reactions. TFA removes these protecting groups, revealing the free amino group for peptide bond formation. It’s like peeling off layers to reveal the masterpiece underneath.
Should You Worry?
TFA is toxic and corrosive. It can cause skin burns and eye damage. However, its use in peptide synthesis is controlled and minimal. Residual TFA levels in peptides intended for preclinical and clinical studies are closely monitored.
Safe Amounts:
TFA concentrations are typically low (e.g., 0.05% to 0.1%) in peptide formulations.
These levels are considered safe for human exposure.
Conclusion
TFA, the unsung hero of peptide chemistry, unlocks the secrets of amino acids and allows us to create intricate peptide structures. While its toxicity warrants caution, proper handling ensures safety. So next time you encounter TFA, remember its vital role in shaping the world of peptides!